Takis and Evvivax developing a 2019-nCoV Coronavirus vaccine
Rome, 27 January 2020 - Takis and Evvivax, two biotechnology companies present in the science park of Castel Romano, Rome announce their commitment for the development of innovative vaccines against 2019-nCoV, the new coronavirus originating in Wuhan, China. To date, the virus has caused 81 deaths and over 2,800 known infections and is rapidly expanding in various countries.
"This is a worldwide emergency to which Takis researchers can and must contribute" - says Luigi Aurisicchio, CEO / CSO of the two companies - "We will immediately make available our skills gained for the development of vaccines against Cancer and other infectious diseases to fight the spread of this coronavirus ". Takis and Evvivax have developed vaccines based on genetic technologies capable of inducing powerful immune responses. "Our technologies are based on genetic engineering techniques and on the use of viruses and DNA fragments that can be used both for gene therapy and for vaccination" - says Emanuele Marra, Director of the Infectious Diseases Area at Takis. “To obtain antibodies capable of neutralizing pathogens, it is essential to use particularly effective technologies. At Takis, thanks to genetic vaccination, we have generated dozens of antibodies capable of neutralizing viruses and pathogenic bacteria "- continues Giuseppe Roscilli, Antibody Area Director. "In various clinical studies conducted in Italy and the USA, our veterinary vaccines have induced a powerful immune response and prolonged the survival of dogs with cancer, which like us humans develop it with high frequency. Even against zoonoses, that is, infectious diseases that are transmitted from animals to humans and vice versa, we can use the same type of approach. "- declares Dr. Antonella Conforti, Director at Evvivax.
Genetic vaccination is therefore the basis for the development of the 2019-nCoV vaccine at Takis. “Coronavirus biology and the availability of the 2019-nCoV genome sequence are all the information we need for vaccine design. The development of the methods and the experimentation will be fundamental to get to human clinical trials as soon as possible "- says Fabio Palombo, Immunology and Cancer Vaccines Area – NeoMatrix Director.
"Thanks to the experience of our scientists, Takis and Evvivax are technologically competitive at an international level and can make their contribution to this Health emergency with the internal resources currently available. However, scientific research requires important investments and it is essential to obtain financing and / or collaborations with large companies as soon as possible that allow us to develop the 2019-nCoV vaccine as quickly as necessary. "- concludes Luigi Aurisicchio.
The race against the virus has started.
For more information: www.takisbiotech.it and www.evvivax.com
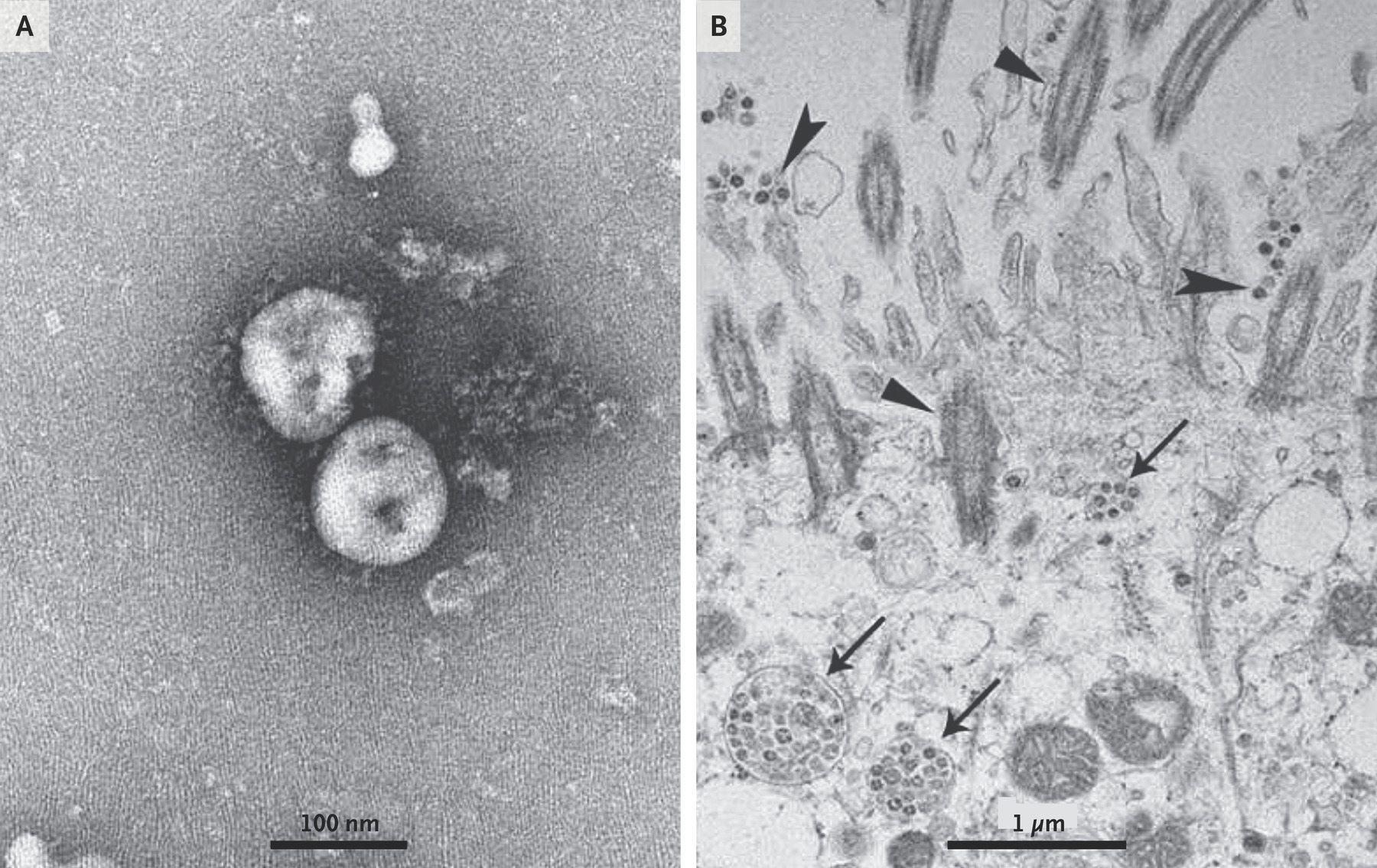
Visualization of 2019-nCoV with Transmission Electron Microscopy.
Negative-stained 2019-nCoV particles are shown in Panel A, and 2019-nCoV particles in the human airway epithelial cell ultrathin sections are shown in Panel B.



















